/ BIOPHARMA COMPANY / IPO – JULY 2019 (NASDAQ: MIRM)
May 8, 2023
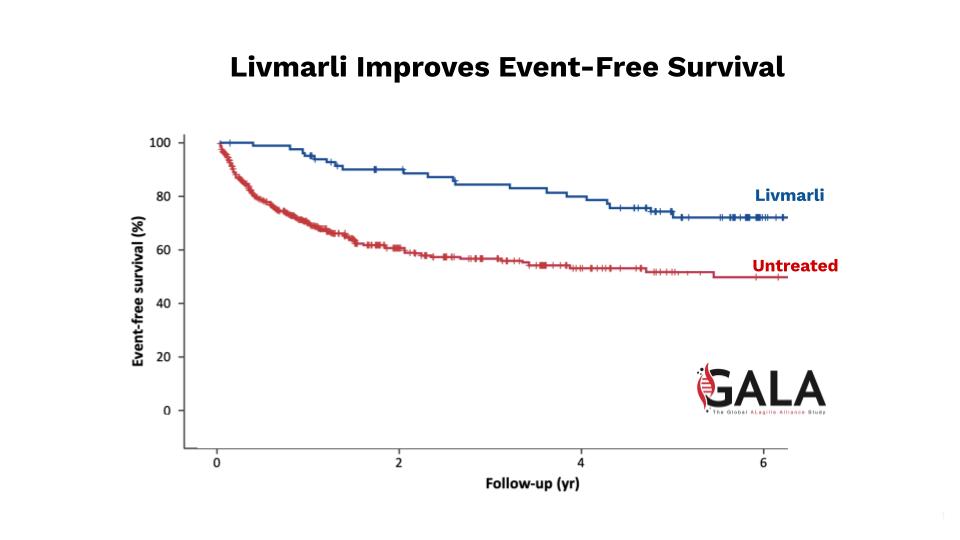
After the drug compound they developed was deprioritized, the Lumena team bought it back so they could finish what they started – getting kids with rare liver disease the relief they need.
THE SNAPSHOT
- $120 million Series A in 2018
- Founded on a treatment co-developed by RiverVest
- IPO: July 2019 (NASDAQ: MIRM)
THE IMPACT
LIVMARLI, a minimally absorbed ileal bile acid transporter inhibitor, is the first and only FDA- approved medication for treating cholestatic pruritus in patients with Alagille syndrome (ALGS) and progressive familial intrahepatic cholestasis (PFIC), rate liver diseases that affect 5,000+ patients in the US and EU.