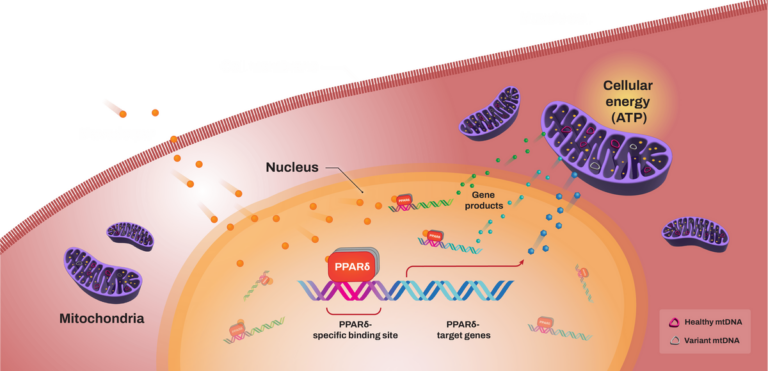
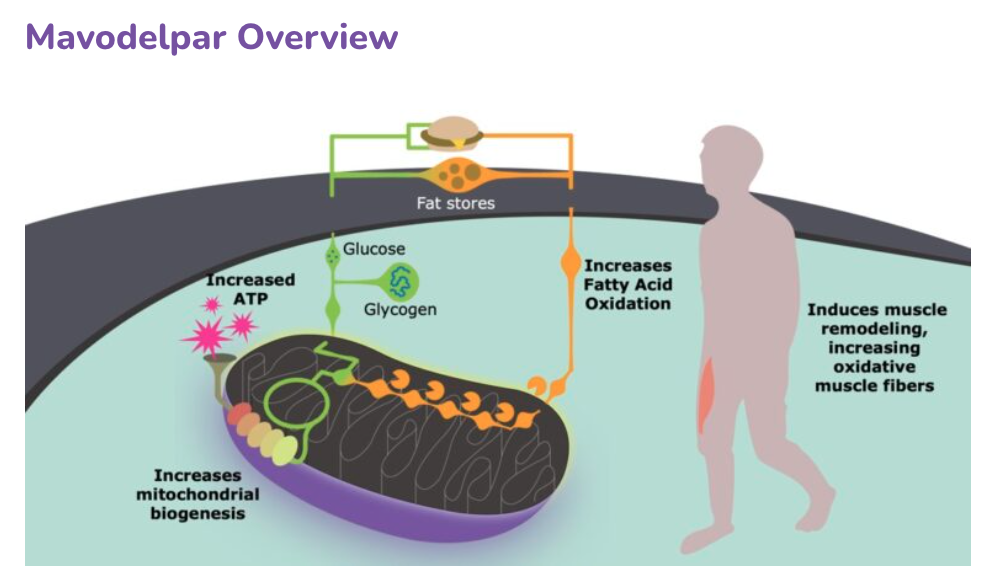
“The key to unlocking mitochondrial disease therapies – or any orphan disease therapy – is establishing symbiotic relationships between drug researchers and patient advocacy groups,” says Wendy Newman, MPH, head of global patient advocacy at Reneo Pharmaceuticals, Inc. “Researchers need empirical data gathered by patient advocacy groups, and patients need access to cutting-edge clinical trials."
Patient advocacy groups such as UMDF facilitate the collection of empirical data through natural history studies. The data, which include both quantitative and qualitative responses from patients and caregivers, should ideally inform every part of the therapy development process.
“Patient-derived data often include quality-of-life priorities that researchers don’t anticipate,” says Yeske. For example, “burden of care” is a leading concern for patients and caregivers but is rarely seen as a primary endpoint in clinical trials. “Drug developers need to understand patient priorities if they want to develop therapeutics that are not only helpful, but also impactful,” says Yeske.
Patient advocacy is a global imperative. Alison Maguire, head of research and finance at the Lily Foundation, the United Kingdom's leading mitochondrial disease charity and the largest charitable funder of mitochondrial research in Europe, stresses the importance of involving the patient early in the research and development process.
"When designing a clinical study, for example, talk to the patients," says Maguire. "Ask them to share their views on whether a study design is practical, if the research is meaningful to them, and if it addresses a real unmet need.”
Like Yeske, Maguire knows firsthand the devastating loss of a child to mitochondrial disease. After her four-year-old daughter Niamh passed away in 2009, Maguire chose to channel her grief and her medical experience into promoting mitochondrial disease research and awareness through the Lily Foundation.
“Remember, it's the patients and their families who are the real experts on mitochondrial disease,” says Maguire. “They live with it every day.”